

The safety and efficacy of the Ga 68 dotatate injection drug product prepared from the NETSPOT kit has been established only when using a Ga 68 chloride solution eluted from the GalliaPharm generator.
#NETSPOT DRUG GENERATOR#
and the European Union is approximately 47,300 patients/year. When executing a search warrant on an East Albany residence Friday, officers from the Albany-Dougherty Drug Unit discovered drugs and weapons, leading to at. GalliaPharm Ge 68/Ga 68 generator (GalliaPharm generator) is not supplied with the NETSPOT kit. The estimated incidence of NETs for the combined populations of the U.S. in two forms: as a kit for reconstitution using a Ga 68 generator, and as Netspot Injection, a ready-to-use dose delivered from a local radiopharmacy in selected metropolitan areas. The product will be available in the U.S. Gallium Ga 68 dotatate received Orphan Drug Designation from both the FDA and European Medicines Agency (EMA) in March 2014.įollowing approval, Netspot will be made available to the U.S. What may interact with this drug This medicine may interact with the following medications: lanreotide octreotide pasireotide steroid medicines like. It is the first approved drug using Ga 68 as a positron emitter. Resources below include materials specific to LUTATHERA (lutetium Lu 177 dotatate), PLUVICTO (lutetium Lu 177 vipivotide tetraxetan), NETSPOT (gallium Ga. Netspot is the new market name for Somakit-TATE (a kit for the preparation of gallium Ga 68 dotatate injection) in the U.S. Netspot received approval following a Priority Review from the FDA. Other drugs that have the same active ingredients. It is based on zedoary and gallium ga 68 dotatate (the active ingredients of Shati and Netspot, respectively), and Shati and Netspot (the brand names). All gathered data will be compared between reported 18F-FDG-PET/CT and NETSPOT imaging for true positive, true negative, positive predictive value and negative predictive value of patient groups.The FDA has approved Netspot (Somakit-TATE) for the localization of somatostatin receptor positive neuroendocrine tumors (NETs) in adult and pediatric patients. Alternative drugs to, pros and cons of the 2 drugs: Shati Netspot (144 reports) How the study uses the data The study uses data from the FDA.
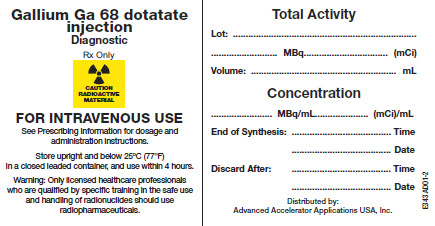
Patients will be followed for a total of 1 year after the study and outcomes of overall survival, disease-free survival, presence and site of recurrence and development of metastatic disease will be recorded. Identify orphan drugs and first-in-class medications approved by the FDA in 2016. Patients with p16-positive tumors will be recorded and analyzed separately for the above measures. Patients with oropharyngeal tumors will have HPV testing performed via the standard of care surrogate marker p16. Biopsies will be performed as is standard to verify all sites of primary or metastatic disease. drug and a gamma camera to measure the radio material over time. Following imaging, both 18F-FDG-PET/CT and NETSPOT imaging will be compared by trained neuroradiologists for SUV values and concordance with known sites of disease. With the creation of the Netspot Scan, we are better able to find these small tumors. For patients undergoing same-day imaging, NETSPOT imaging will be performed prior to 18F-FDG-PET/CT. NETSPOT is the new market name for Somakit-TATE (a kit for the preparation of gallium Ga 68 dotatate injection) in the US. These scans will be performed no less than 8 hours, no more than 7 days apart. The NETSPOT kit is supplied as 2 vials see Dosage Forms and Strengths which allows for direct preparation of Ga 68 dotatate injection with the eluate from one of the following generators (see below for specific instructions for use with each generator): Eckert & Ziegler GalliaPharm Germanium 68/Gallium 68 (Ge 68/Ga 68. Following 18F-FDG-PET/CT, the patient will undergo NETSPOT imaging with the AAA-provided dose. All patients will undergo 18F-FDG-PET/CT per established protocol. Patients meeting inclusion criteria will be consented at the time of scheduling. Why Should I Register and Submit Results?.


 0 kommentar(er)
0 kommentar(er)
